How it all started
The distinction between thermodynamic and kinetic stability of a compound or a chemical bond was the driving force to start organometallic thermochemistry studies in Lisbon, in 1975. At that time there was a considerable debate in the literature about the "strength" of transition metal-carbon bonds. Homoleptic species (MRn)x containing transition metals (M) bonded to σ-hydrocarbyl ligands (R) seemed to be very difficult to prepare and the known compounds showed a high thermal instability (e.g. tetramethyltitanium (TiMe4) decomposes a few degrees above -78ºC and hexamethyltungsten (WMe6) decomposes explosively at room temperature). In contrast, numerous (MRn)x derivatives of the main group elements were stable at room temperature.
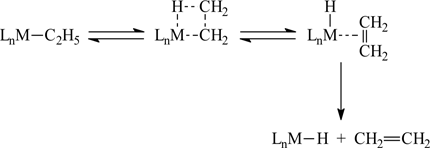
Those observations had led to the general belief that transition metal-carbon bonds were weak and therefore the instability of the transition-metal σ-hydrocarbyls had a thermodynamic origin. This view was challenged, however, by the groups of Wilkinson and Lappert, who, in the early seventies, independently suggested that the instability of the transition metal σ-hydrocarbyls had a kinetic origin. These authors pointed out that β-elimination (see scheme) was an important pathway for the thermal decomposition of transition metal σ-hydrocarbyls and that stable complexes could often be prepared by using ligands without β-hydrogen atoms (e.g. Me3CCH2, Me3SiCH2, and PhCH2). As observed in the scheme, the destabilisation of the four-centre transition state, due to the stretching of the metal-carbon bond, is compensated by the formation of an incipient metal-hydrogen bond. The pathway does not require the simple cleavage of the M-C σ bond. Hence, the thermodynamic stability of this bond could not be inferred from the decomposition temperature of the compound.
The controversy motivated Alberto Romão Dias (1941-2007), head of the Organometallic Chemistry group at Centro de Química Estrutural - Instituto Superior Técnico, and Jorge Calado (b. 1938), head of the Experimental Thermodynamics group at the same institution, to investigate the magnitude and trends of transition metal-ligand bond dissociation enthalpies. One of the conclusions of joint discussions was the decision to hire a graduate student to work on the project (José Artur Martinho Simões, b. 1952). The studies were to be centered on bis(η5-cyclopentadienyl) complexes, MCp2Ln (M = Ti, Zr, Hf, V, Nb, Ta, Mo, W; L = H, halogen, CO, alkyl, alkene, aryl, alkoxide, thiolate, phosphine, etc.; n = 1-3) for two main reasons: (i) the know-how for their preparation was available at CQE-IST, since all the synthetic work of the organometallic chemistry group was based on these systems; (ii) bis(η5-cyclopentadienyl) complexes seemed ideal to probe trends in bond dissociation enthalpies since it was possible to prepare series of compouds of a given metal with various ligands and vice-versa. After a number of exploratory experiments, involving several techniques, it was established that reaction-solution calorimetry was the method of choice to study the energetics of the M-L bonds in MCp2Ln complexes. Since most bis(η5-cyclopentadienyl) compounds were oxygen-sensitive, it was also necessary to develop an anaerobic calorimeter.
Two groups from outside CQE-IST gave a significant contribution in the first steps of the experimental studies. A group from the University of Manchester (UK), headed by Henry Alistair Skinner (1916-1996), Geoffrey Pilcher (b. 1927), and Joseph A. Connor, who were the leading authorities on thermochemistry of organometallic compounds; and the group of Manuel Ribeiro da Silva (b. 1940), at the University of Porto (Portugal) whose main interests were in the thermochemistry of coordination complexes.
José Artur Martinho Simões obtained his PhD in 1981 and, after a post-doc at California Institute of Technology, USA, with Jack Beauchamp (b. 1942), returned to IST where he supervised (together with Professor Romão Dias) a number of PhD students. In 1988-1989 he spent a sabbatical year at National Research Council of Canada (Ottawa), where he learned how to use photoacoustic calorimetry. In 1993 he moved to Faculdade de Ciências, Universidade de Lisboa, and later founded the Molecular Energetics Group.
The Molecular Energetics Group is now a network of chemists in the Lisbon area who are interested in the field. It includes members and associate members from several institutions.
(Adapted from Journal of Organometallic Chemistry 2001, 632, 188-196)
