Case studies
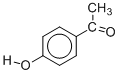
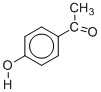
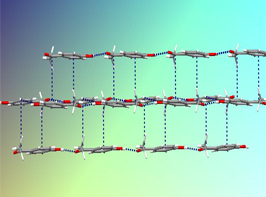
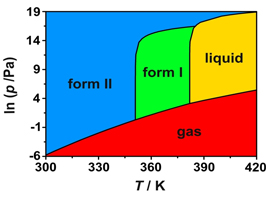
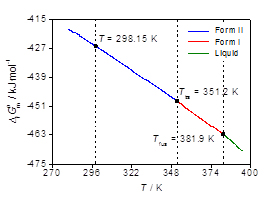
The questions identified in the introduction are most conveniently addressed within the scope of thermodynamics as illustrated by a study of 4'-hydroxyacetophenone (HAP) [6]. As shown in Figure 1 two anhydrous polymorphs were isolated which differ in their crystal structure and morphology, and in physical properties (e.g. density and fusion temperature). The conformation of the HAP molecule is also distinct in both forms (conformational polymorphism). The corresponding pressure vs. temperature and Gibbs energy vs. temperature diagrams (Figure 2) could be determined by combining the results of a variety of methods: combustion calorimetry, solution calorimetry, Calvet-drop calorimetry, differential scanning calorimetry vapor pressure measurements by the Knudsen effusion technique and computational chemistry calculations [6].
Form I T |
Form II T |
 |
 |
 |
 |
 |
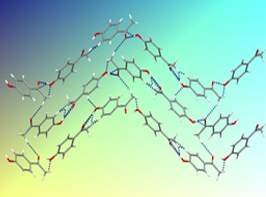 |
Figure 1. Forms I and II of 4'-hydroxyacetophenone.
 |
 |
(a) |
(b) |
Figure 2. (a) Pressure vs. temperature and (b) Gibbs energy vs. temperature diagrams for the 4'-hydroxyacetophenone system.
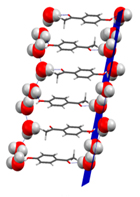
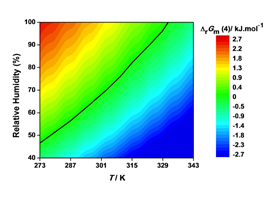
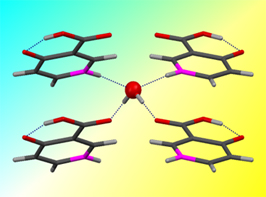
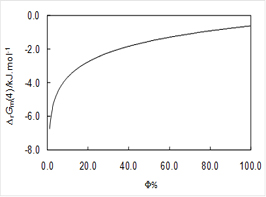
Co-crystals and solvates can also decompose leading to different solid phases. It is, for example, very common for a hydrate to transform into an anhydrous form by losing water when changes in ambient pressure, temperature, and humidity occur. One of the most succinct ways of presenting the stability domains of hydrates is in terms of the Gibbs energy change upon dehydration as a function of temperature and relative humidity. This is illustrated in Figure 3 for and hydrate of 4'-hydroxyacetophenone of stoichiometry HAP•1.5H2O [7] and in Figure 4 for an hemyhydrate of 4-hydroxynicotinic acid (4HNA) [8] that were isolated in our laboratory. In the first case the water molecules are arranged in channels (channel hydrate) while in the second case they are isolated from each other.
 |
 |
 |
(a) |
(b) |
(c) |
Figure 3. (a) Crystal packing of the hydrate phase of 4'-hydroxyacetophenone (HAP•1.5H2O) showing the water chains parallel to the (002) plane. (b) SEM image of the crystals of HAP•1.5H2O. (c) Gibbs energy change upon dehydration of as a function of temperature and relative humidity.
 |
 |
(a) |
(b) |
Figure 4. (a) Local structure of 4HNA•0.5H2O around a water molecule. (b) Gibbs energy change upon dehydration of 4HNA •1.5H2O at 298.15 K as a function of relative humidity.
