The energetics and structure of ionic liquid aqueous solutions
Some ionic liquids are considerably soluble in water, and their aqueous solutions therefore became the subject of a variety of potential applications [35-38]. This also fostered various theoretical and experimental studies on the structure and energetics of those solutions. Most of them were focused on ILs of the 1-3-dialkylimidazolium family. From these studies a general picture emerged where in the limit of very low concentrations of water, the H
Of particular interest to us was the relationship between the structure and energetics of aqueous IL solutions. This motivated the study of 1-ethyl-3-methylimidazolium ethylsulfate, [C
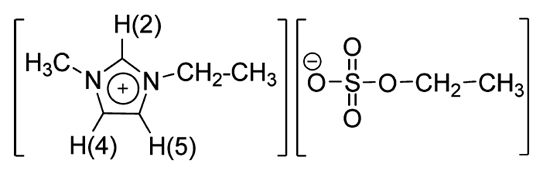
Figure 1. Molecular structure a of the 1-ethyl-3-methylimidazolium ethylsulfate, C2mim][EtSO4], ionic liquid.
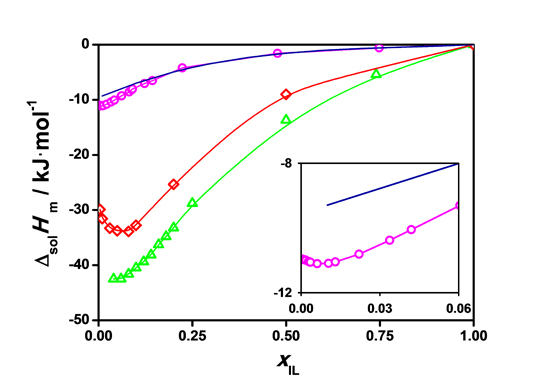
Figure 2. Molar enthalpies of solution of [C2mim][EtSO4] in water (per mol of IL) at 298.15 K as a function of the ionic liquid molar fraction. Magenta solid line, our work experimental data. Red solid line, our work molecular dynamics simulation data. Blue solid line and Green solid line, previous experimental and molecular dynamics data.
The separation of our values obtained by MD simulations into contributions from IL-IL, H
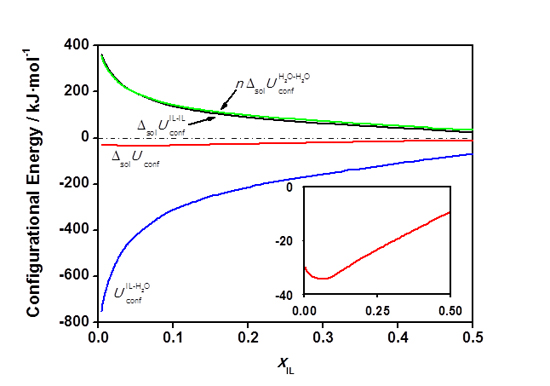
Figure 3. Configurational internal energy of solution, ![]() , calculated from MD simulations (red line) as a sum of contributions from different types of interaction. All internal energy quantities are expressed in kJ per mol of ionic liquid.
, calculated from MD simulations (red line) as a sum of contributions from different types of interaction. All internal energy quantities are expressed in kJ per mol of ionic liquid.
The analysis of the MD simulations results using different in-house developed analysis tools to describe the size and connectivity of different water and ion aggregates as a function of the solutions concentration also allowed the identification of four concentration ranges where four distinct structural regimes are present (Figures 4 and 5): isolated water molecules (x
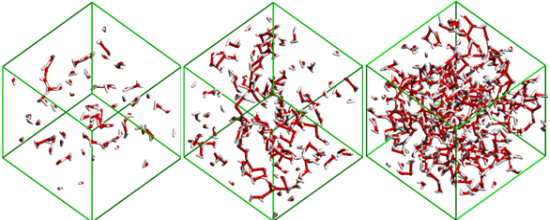
Figure 4. Snapshot images of the water clusters in simulation boxes with a) xH2O=0.5, b) xH2O=0.8 and c) xH2O=0.92. The pictures show that when small amounts of H2O molecules are present they are either isolated or form chains around the polar network of the ionic liquid (not represented). When the water percolation limit is attained (between b) and c)), the water molecules form a continuous branched network surrounding the ionic liquid.
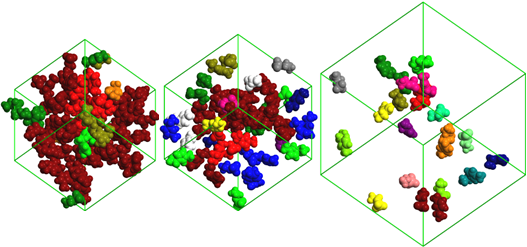
Figure 5. Snapshots of the ionic liquid aggregates in simulation boxes with a) xH2O = 0.95, b) xH2O = 0.97 and c) xH2O = 0.996. Ions of the same color belong to the same aggregate. The pictures show that at xH2O = 0.95 the IL network starts to break down and multiple aggregates are formed. Finally, when just a few IL pairs are present at xH2O = 0.996, only isolated ions and dimmers are found in solution.