Autoxidation and antioxidation
It well know that fats become rancid, even when kept at low temperatures. This is due to a reaction between lipid molecules and molecular oxygen, which is called autoxidation or lipid peroxidation. This reaction can be hindered by using substances known as antioxidants. For instance, the shelf-live of many foods is considerably extended by the addition of suitable antioxidants. Paints, rubber, and plastics, for example, also undego autoxidation and therefore need protection.
The membranes of living human cells also contain lipids, most of which are phospholipids based on glycerol and polyunsaturated fatty-acid side-chains (see Figure 1). Therefore, they should undergo autoxidation. The fact that they do not, means that they have some protection system.
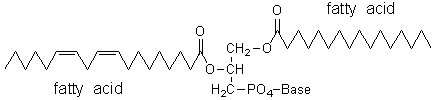
Figure 1
The phospholipids are essential constituents of the cell membranes. They form the so-called lipid bilayer, illustrated in Figure 2 (adapted from B. Halliwell, J. M. C. Gutteridge, Free Radicals in Biology and Medicine; 2nd ed.; Oxford University Press: Oxford, 1989). The phospholipid molecules arrange themselves so that the non-polar "tails" (the unsaturated carbon chains) are away from the aqueous phase, and the polar "heads" (the phosphate group; yellow circles) are turned to the surrounding water molecules. The other indicated constituents of the membranes are proteins (blue forms), cholesterol molecules (red triangles; see Figure 3), particularly important in plasma membranes, and vitamin E (green triangle).
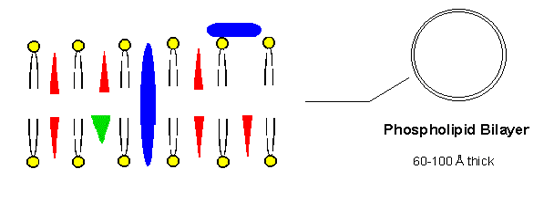
Figure 2
Figure 3
The lipid peroxidation is a chain reaction, which means that it consists of several interconnected steps where the products of some are the reactants of others. The first step is called initiation and in the case under discussion it involves the formation of a (carbon centered) lipid radical. This is often achieved by hydrogen abstraction from the lipd molecule (LH) by an hydroxyl radical, OH•:
| LH + OH• → L• + H2O | initiation |
The most probable site of attack for the hydroxyl radical is the —CH2— group that bridges the two double bonds of the chain (see Figure 1), simply because this will correspond to the weaker C—H bond (the radical so formed is resonance-stabilized by a bis-allylic arrangement). The radical L• can undergo several reactions (e.g. it can cross-link with other radical in the same molecule), but the most probable will be its reaction with molecular oxygen dissolved in the cell membrane, yielding the peroxyl radical LOO•:
| L• + O2 → LOO• | propagation |
The peroxyl radical can then abstract an hydrogen atom from another molecule of lipid (LH), forming a new lipid radical,
| LOO• + LH → LOOH + L• | propagation |
which can then react with oxygen, and so on. Although the chain can be broken by a termination step,
| LOO• + LOO• → molecular products | termination |
the production of oxidized species is very efficient. Fortunately, the second propagation step is relatively slow, which allows to break the chain by using an antioxidant such as α-tocopherol (TocH; see Figure 4, adapted from Nagaoka et al., J. Phys. Chem. B 2000, 104, 856). This molecule reacts with the peroxyl radical LOO• and therefore prevents the formation of a new lipid radical L•. Since α-tocopherol breaks the oxidation chain reaction it is called a chain-breaking antioxidant. As noted in Figure 4, TocH can be regenerated through the action of vitamin C (AH-; ascorbate) at the membrane interface with the aqueous solution or with the ubiquinol (UQH2; Figure 5), within the cell membrane.
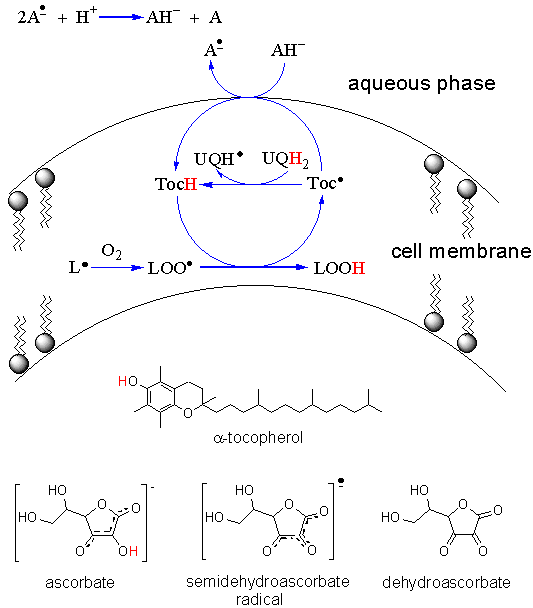
Figure 4
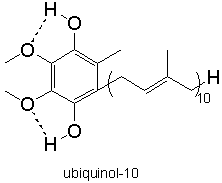
Figure 5
Chain breaking antioxidants play a very important role in our lives. There are many natural substances, contained in food and beverages like chocolate (cocoa), tea, and red wine, which contain many of these substances.
