Crystallization
Crystallization from solution is by far the most widely used method for the preparation of a product corresponding to a specified crystalline form with consistent and well-characterized physical properties. It is nevertheless frequent that new solid forms are obtained in the course of crystallization screening tests without tight control of the experimental conditions. In these cases the preparations may be difficult to replicate and this has motivated numerous efforts to develop systematic methodologies for the selective and reproducible production of polymorphs, solvates, and co-crystals [1-3]
If tight control over selective crystallization of the different forms is to be achieved, then a reasonable understanding of their nucleation and crystal growth is necessary. A key element towards this goal is a temperature vs. concentration phase diagram illustrating the saturation and supersaturation limits (metastable zone width) of the solution in the compound of interest, and the stability domains of the different crystallized phases. It should be kept in mind that since nucleation requires some degree of supersaturation (a metastable state) the crystallized phases may not always be the most thermodynamically stable ones. Depending on the kinetic barriers for transformation metastable phases may quickly evolve into more stable forms (low barrier) or stay unchanged almost indefinitely (high barrier). One such phase diagrams obtained for 4'-hydroxyacetophenone by using a fully automatic crystallization reactor (CB1 apparatus) [4] is illustrated in Figure 5 [5]. It allowed a convenient definition of the experimental conditions for the selective production of the anhydrous forms I and II or the HAP•1.5H2O hydrate.
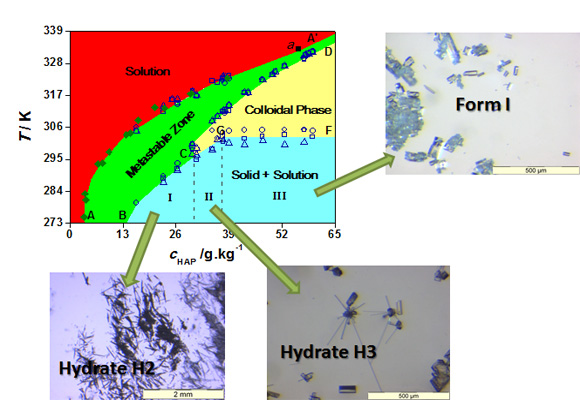
Figure 5. Phase diagram of the HAP + water system. The concentration of HAP, cHAP, refers to grams of anhydrous solute per 1 kg of water.
