Ionic liquids
Ionic liquids are a remarkable class of Coulombic fluids in which interest has burgeoned in latest years, due to the challenges and opportunities offered in terms of fundamental research and technological applications [1-4]. The work of the Molecular Energetics group in the ionic liquid field has involved collaborations with researchers from various institutions, and has been essentially centered on:
- The study of ionic liquids vaporization.
- The energetics and structure of ionic liquids aqueous solutions.
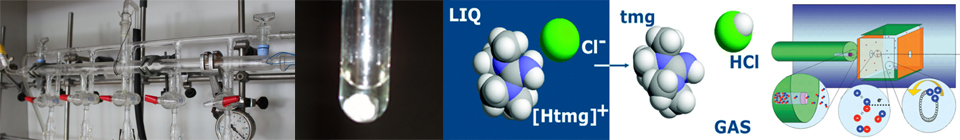
Ionic liquids are normally regarded as organic salts which, conventionally, melt below 373 K and may be considered as either protic or aprotic in character. Protic ionic liquids (PILs) are formed by proton transfer from a Brönsted acid, AH, to a Brönsted base, B to yield, strictly speaking, a [BH+][A-] species [5]:
| AH + B = [BH+][A-] | (1) |
There is, however, a grey area associated with the practical application of this definition. In order for [BH+][A-] to be commonly considered as a pure liquid, the amounts of the neutral AH and B precursors involved in the equilibrium stated by eq 1 should be negligible. It has therefore been proposed, as a guideline, that a pure protic ionic liquid should contain less than 1% of the neutral species [6]. Significant proton transfer must also occur if the liquid is to be considered ionic, otherwise the product of the reaction of AH with B will be better described as an adduct sustained by a AH...B hydrogen bond. It was recently discussed that, actually, the underlying structure can be even more complex - for instance, the possible existence of multiple equilibria leading to the formation of electrically charged aggregates [7]. Nonetheless, serious efforts have been undertaken to quantify the extent of proton transfer and the ionicity of PILs by using, for example, differences in the aqueous pKas of AH and BH+ [8-10], ionic conductivities in the form of Walden plots [8,9], and data from 1H-NMR [10-12], vibrational spectroscopy [12], or cyclic voltammetry experiments [13]. The matter, however, has yet to be settled [5,12].
Aprotic ionic liquids (AILs) contain substituents other than a proton (typically an alkyl group) at the site occupied by the labile proton in an analogous protic ionic liquid. In this case an equilibrium such as that expressed in eq 1 cannot be established, thus removing the ambiguities of definition mentioned above for PILs. They also require synthetic strategies which are different from the simple acid-base reactions used to obtain most protic ionic liquids [14].