Aprotic ionic liquids were initially considered as essentially involatile, but this belief has been shown to be incorrect, and it is now well established that many ILs of this type can be distilled under reduced pressure without decomposition [15]. The vapor pressures and/or enthalpies of vaporization of some of them have also been experimentally determined by various methods including Knudsen effusion [16-18] transpiration [19], mass spectrometry [20], calorimetry [21] and thermogravimetry [22]. A critical review of these studies has recently been published [23].
We previously investigated the nature of the species present in the gaseous phase during the reduced pressure distillation of aprotic ionic liquids using Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS) [24]. The results indicated that the uni-univalent AILs, 1-methyl-3-alkylimidazolium bis{(trifluoromethyl)sulfonyl}amide, [C
nmim][NTf2] (n = 2, 4, 6, 8, or 10), 1-methyl-3-butylimidazolium hexafluorophosphate, [C4mim][PF6], and trihexyltetradecylphosphonium trifluoromethyl sulfonate, [P(C14H29)(C6H13)3
][OTf] (Figure 1), distil under reduced pressure (10-6 - 10-4 Pa) as neutral anion-cation pairs. This conclusion corroborated the mass spectrometric results of Armstrong et al. [20] for the evaporation of thin films of aprotic ionic liquids into ultra-high vacuum, and has subsequently been confirmed by more recent work [25,26] Furthermore FT-ICR-MS studies of the binary mixtures ([C4mim][NTf2] + [C4mim][PF6]) and ([C4mim][NTf2] + [P(C14H29)(C6H13)3][OTf]) indicated the occurrence of fractional distillation [24]. This type of studies was very recently extended to a dicationic AIL 1,3-bis(3-methylimidazolium-1-yl) propane bis(trifluoromethanesulfonyl)imide, [C3(mim)2][NTf2]2 Figure 1), formed by combination of a divalent cation with two univalent anions. The results showed that, analogously to the uni-univalent AILs, in this case, the vapor is also composed solely by the parent neutral ion triplet (NITs) e.g. the triplet formed by one dication and two anions [27].
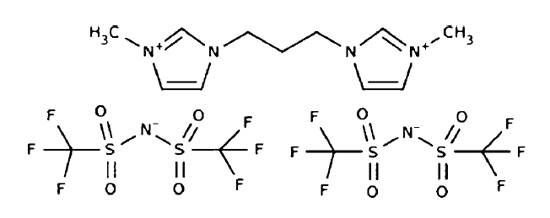
Figure 1. 1,3-bis(3-methylimidazolium-1-yl) propane bis(trifluoromethanesulfonyl)imide.
We also examined the distillation of molten salts composed of a Group 1 metal cation and a commonly used ionic liquid anion, M(NTf2) (M = Li, Na, K, Rb, Cs), at 600 - 680 K and 6×10-6 - 2×10-4 Pa. It was concluded that similarly to the studied aprotic ionic liquids, the vapor phase essentially consists of neutral ion pairs, although, in the case of the lithium and sodium derivatives, a minor contribution from [M2(NTf2)2] neutral aggregates was observed [28]. These substances therefore exhibit a behavior somewhat intermediate between that of aprotic ionic liquids and Group 1 metal halides, MX (M = Li, Na, K, Rb, Cs; X = F, Cl, Br, I). Vapors originating from the latter under low pressure show significant fractions of aggregates larger than ion-pairs (particularly dimers), with the tendency for aggregation decreasing as the sizes of the M+ or X- ions increase (e.g. LiCl > NaCl > KCl > RbCl > CsCl and NaF > NaCl > NaI) [29-31].
Because of the equilibrium stated in eq 1, protic ionic liquids tend to vaporize by an intrinsically different mechanism from those of aprotic ILs, M(NTf2), or MX salts, decomposing into the two neutral acid and base precursors [15]:
| [BH+][A-](l) = AH(l) + B(l) → AH(g) + B(g) |
(1) |
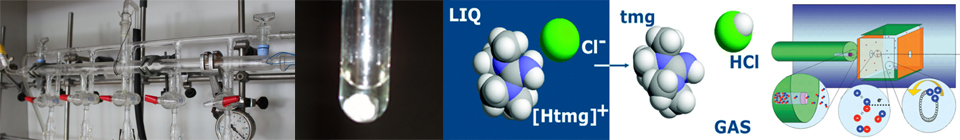
This tendency seems, however, to significantly depend on the nature of these precursors and on the “strength” of the donor acceptor interaction [8,9,12]. FT-ICR-MS experiments on 1-methylimidazolium ethanoate, [Hmim][O2CCH3
] [24], and on 1,1,3,3-tetramethylguanidinium chloride, [Htmg]Cl [32] carried out in our laboratory suggest that, as previously assumed, PILs tend to vaporize under reduced pressure with the formation of the two neutral precursors AH and B in eq (1) [24,32-34]. There is also a tendency of these precursors to become associated in the gas phase by means of reaction (1). The extent of this process strongly depends on the pressure and on the nature of the acidic and basic moieties [24,32-34].