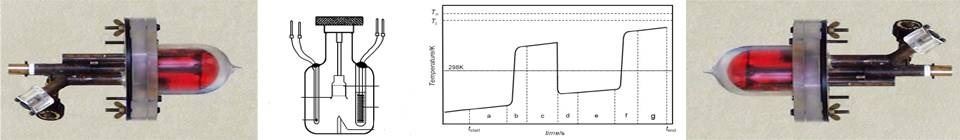
The reaction-solution calorimeter to determine bond strengths
One of the more commonly studied reactions in a reaction-solution calorimeter is the ligand exchange reaction. On such a reaction two species react to give another two species by changing a ligand. As an example the reaction of an alkaline metal alkoxide (MOR) with water is presented:
| MOR + H |
(1) |
During this process two chemical bonds are cleaved and two other formed. Representing a bond dissociation enthalpy (DH) as the enthalpy associated with a reaction like:
| ROH = RO• + H• | (2) |
the enthalpy change of the first reaction (Δ
| Δ |
(3) |
If three of these BDEs are known, then the other one can be calculated.