Reaction-Solution Calorimetry
Two different types of experiments can be used to obtain the enthalpy of solution for the mixture of two substances: an isoperibol solution calorimeter or a heat conduction calorimeter.
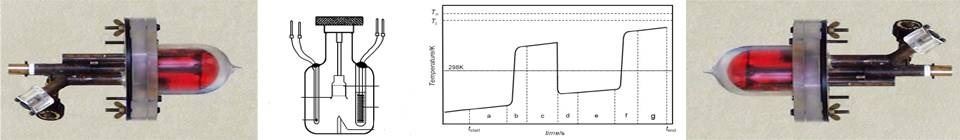
The principle of an isoperibol solution calorimeter is simple. A liquid reactant is contained in a Dewar, which is itself immersed in a thermostatic bath, usually kept at 298.15 K. Another reactant, either a liquid or a solid, is put in a small glass cell and immersed in the first liquid. Mixing is achieved mechanically. After the system comes to thermal equilibrium, the reaction is started by breaking the glass cell, to release the reactant inside the Dewar cell. The heat released or absorbed causes the temperature of the system to rise or drop, depending on the exo/endothermicity of the process. The reaction is allowed to go to completion and the temperature change is measured.
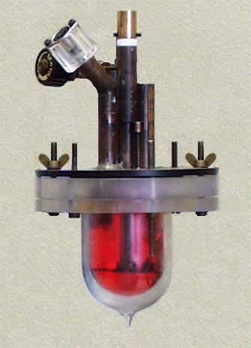
Figure 1. Dewar cell for anaerobic reaction-solution calorimetry.
The heat capacity of the solution calorimeter is calibrated by studying the reaction of a standard system. Commonly, the neutralization of TRIS (tris(hydroxymethyl)aminomethane) by HCl is used to standardize a solution calorimeter. Once the heat capacity of the calorimeter is known, any process in solution (reaction, mixing, precipitation, etc.) can be determined. The amount of heat absorbed by or removed from the solution can be calculated by the following equation.
| q = c m ΔT | (1) |
where q represents the amount of heat transferred, c the specific heat of the solution, m the mass of the solution, and ΔT the temperature change associated with the process under study. A typical record for a exothermic process is shown below.
In heat conduction (or heat flow) calorimeter, heat is completely exchanged by conduction between the calorimeter proper and the jacket. In practice, the thermal conductivity of the interspace is made large by placing a thermopile between the calorimeter proper and the jacket. In a solution calorimetry experiment, the jacket temperature is constant and accurately controlled with a precision < 0.1 mK. The jacket has a very high heat capacity compared with that of the calorimeter proper and acts as a heat sink, that is not affected by the total amount of thermal energy being transferred.
As described for the Calvet-drop microcalorimeter experiments, the enthalpy of the process is computed from the area of the measuring curve obtained for the investigated process and converted into energy by using the calibration constant determined by Joule effect. At the molecular energetics group, these experiments are performed by using an LKB 2277 thermal activity monitor (TAM; Figure 2).

Figure 2. LKB 2277 thermal activity monitor used in solution calorimetry experiments at the Molecular Energetics Group.