Equipment
All FT-ICR-MS instruments have four common components: a magnet (which is responsible for its resolution, because it increases with the magnetic field strength), the analyzer cell (where ions are stored, mass analyzed and detected), an ultra-high vacuum system (which improves the resolution of ion detection) and a data system (which includes a frequency synthesizer, delay pulse generator, broadband r.f. amplifier and pre-amplifier, a fast transient digitizer and a computer to coordinate all of the electronic devices during the acquisition of data, as well as to process and analyze the data).
The ion motion in the cell is complex because of the presence of electrostatic and magnetic trapping fields, consisting of three different modes of oscillation (cyclotron, magnetron and trapping) [2]. However FT-ICR-MS technique can be based on the classical motion of ions described by elementary laws of electromagnetism [3]. The electrostatic and magnetic forces acting on the ion can be described by Lorentz equation:
| F = m a = q (E + vB) | (1) |
where the force, F, equals the acceleration, a, acting on an ion of mass, m. The trajectory of an ion with charge q is dependent on its initial velocity, v, the electric field, E, and magnetic field, B. In the absence of an electric field and assuming that no ion-molecule collisions occur, it was demonstrated that a charged particle moving perpendicular to a uniform magnetic field is constrained to a circular orbit in which the angular frequency of the particle's motion is independent of the particle's orbital radius [4] and the frequency cyclotron frequency) is related to the ion mass by the fundamental cyclotron equation:
| (2) |
The cyclotron motion of a particle could be excited to a larger orbital radius by applying a transverse alternating electric field whose frequency matched the cyclotron frequency of the particle. Thus a particle could be excited to very large kinetic energy by use of only a modest electric field strength. Experimentally, ions are produced between two trapping plates perpendicular to the magnetic field, to impose an electrostatic field that constrains ions motion along the z-axis and to prevent ions to be lost. These plates are maintained at a repulsive potential typically +1V or -1V for positive and negative ions respectively (Figure 2) [5].
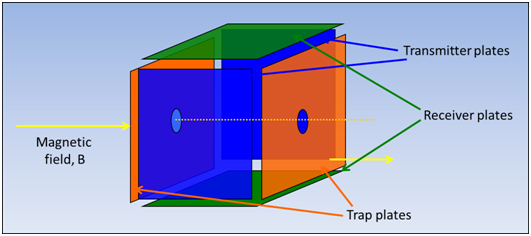
Figure 1. Representation of the FT-ICR-MS cell.
In our specific case, the FT-ICR-MS cell, a trapped ion cell, is composed by a box-shaped cell of six plates, which allows ion formation, manipulation and detection to occur during discrete temporal events within the same volume in the space [6]. The excitation and detection of the trapped ions require two sets of plates lying along the axis of the magnetic field between trapping plates (by convention in Cartesian coordinates, the z-axis is collinear with the magnetic field, the x-axis is perpendicular to excitation electrodes and the y-axis is perpendicular to the detection electrodes) [7]. These plates are the transmit and the receive plates. This cell is mounted in a high vacuum chamber in a strong magnetic field. The ions trapping efficiency depends on magnetic field strength and pressure; the later determines the number of collisions per unit of time between the ions and the neutral molecules [8]. Improved vacuum conditions result in an elongated mean free path for the ions and thus reduce the risk of destabilizing collisions between ions and residual neutral molecules. The main vacuum chamber containing the analysis cell is pumped by a large diffusion or turbomolecular pump, in order to obtain a total pressure on the 1.33 x 10-7 - 1.33 x 10-4 Pa range [9].
Application of an external oscillating electric field across the transmit plates at the characteristic cyclotron frequency of an ion causes ions of that mass to charge ratio to move into resonance with the applied field and spiral out to larger radius orbits. As the ions move into resonance with the electric field, their motion is shifted from having a random distribution of phase to a "packet" of ions, in which all ions simultaneously move in phase. Experimentally, this induction of coherent motion is accomplished by applying a broadband radio frequency "chirp" pulse, a well-defined SWIFT (stored waveform inverse Fourier transform) pulse or a voltage impulse strike [10]. The image current is converted to a voltage, amplified, digitized by an analog-to-digital converter and stored in a computer. This cycle is repeated many times to improve the signal-to-noise ratio by signal averaging. A Fourier transform is applied to the stored data to analyze any complex time domain signal to yield a frequency spectrum that contains complete information about frequencies and abundances of all ions trapped in the cell [11].
The excitation of the ion's cyclotron motion allows the detection of ions, because the radius of an ion's cyclotron orbit when first trapped is usually small when compared with the dimensions of the cell. Therefore, the ions are excited into coherent motion by applying a sinusoidal voltage to the excitation plates which generate a mass-independent radius on the ion cell [12]. Ions that are not in resonance do not absorb energy and remain at the center of the cell. As the ions pass the cell's electrodes, the coherently orbiting ion packet attracts electrons to first one and then the other of the two detection plates through the external circuit that joins them. This alternating current is called the image current, which is non-destructive, because the ions remains in the analyzer cell after the detection process has been completed [13].
The vacuum system is typically maintained at a pressure of 1.33 x 10-4 Pa or lower so that collisions happen infrequently. However for a variety of experiments, the pressure may be raised. Under these conditions, collisions will occur between ions and neutral molecules, which cause a decrease in the kinetic energy of the ions and therefore a reduction of their velocity [13]. Also, intentional and controlled collisions can be used to study chemical reactions between ions and neutral molecules inside the cell.
Therefore in a FT-ICR-MS instrument, the principal functions of ionization, mass analysis and ion detection to occur in the same space (the analyzer cell), but these events are spread out in time. The experimental sequence is composed by quench, ion formation, if wanted ion reaction, and finally ion excitation and detection [13].
