Examples
Kinetic and equilibrium studies of ionic liquids
[C2mim]+ + [C2mim][NTf2] = [C2mim]2[NTf2]+
V = kf[A+][AB] = kr [A2B+]
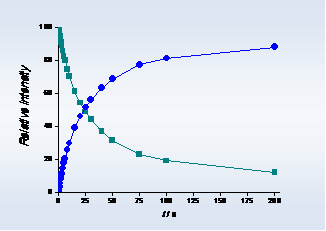
(a)
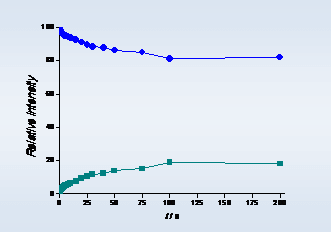
(b)
Figure 1. (a) Association reactions between [C2mim]+ (-■-) and [C2mim][NTf2] and (b) dissociation reaction of [C2mim]2[NTf2]+ (-●-) in gas phase, respectively. T = 298 K; PArgon= 2.6 x 10-4 Pa.
Determination of the gas phase of 1,1,3,3-tetramethyliguanidinium chloride [14]
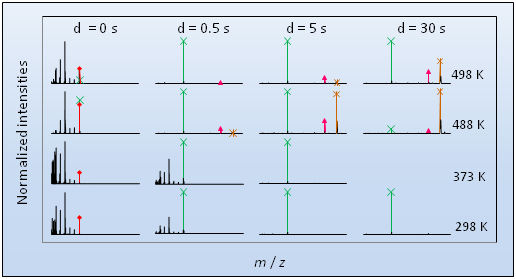
Figure 2. FTICR-MS spectra of [Htmg][Cl] subliming/vaporizing at different temperatures and delays with a nominal argon pressure of 5.3×10-6 Pa: [tmg]+ (m/z = 115) ◊, [Htmg]+ (m/z = 116) ×, [H(tmg)2]+ (m/z = 231) ▲ and [(Htmg)2Cl]+ (m/z = 267) *.
Vaporisation of a Dicationic Ionic Liquid [15]
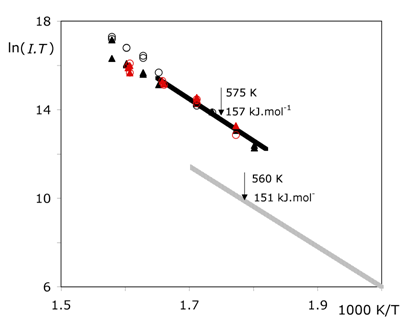
Figure 3. Clausius-Clapeyron-type plot of the normalized peak intensities of {[C3(mim)2][NTf2]}+ at m/z 486 (▲) and of [CH2=CHCH2mim]+ at m/z 123 (●).
