Heat flow calorimetry
Heat flow calorimeters, also known as “heat flux,” “heat conduction,” or “heat leakage” calorimeters, are instruments where the heat output or input associated with a given phenomenon is transferred between a reaction vessel and a surrounding thermostat jacket. The temperature of the jacket is kept constant and is to a good approximation unaffected by the heat exchange with the reaction cell. The jacket is therefore designed to function as a heat source/sink of virtually infinite heat capacity.
The heat transfer between the reaction vessel and the jacket can be monitored with high thermal conductivity thermopiles containing large numbers of identical thermocouple junctions regularly arranged around the reaction vessel (the cell) and connecting its outside wall to the heat source/sink (the thermostat).
The determination of the heat flow relies on the so-called Seebeck effect. An electric potential, known as thermoelectric force is observed when two wires of different metals 1 and 2 (Figure 1) are joined at both ends and these junctions are subjected to different temperatures, T
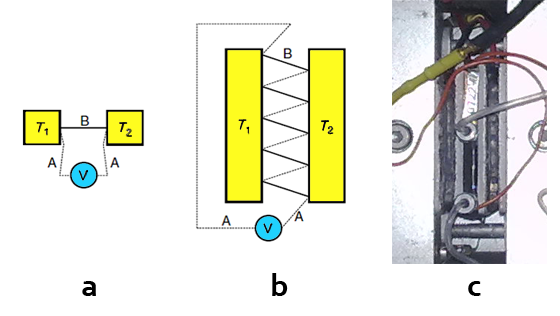
Figure 1. A thermocouple (a) and a thermopile (b) as devices for measuring a temperature difference or a heat flow. A and B are wires of different metals (adapted from ref [1]). (c) Picture of the LKB microcalorimeter thermopile.
Typically, a heat flow calorimetry experiment is performed as follows: (i) initially the calorimetric cell and the jacket are in equilibrium and therefore T
Currently, there are two different types of Heat Flow Calorimeters at the Molecular Energetics Group:
