The photoacoustic calorimeter is also a tool for determining rate constants
As exemplified in the Bond Dissociation Energies chapter, by using BDEs alone we can predict the outcome and efficiency of many chemical reactions. However, as any undergraduate organic chemistry student would point out, there are many cases in which we… cannot. Quite appropriately, reactions of the first kind are called thermodynamically controlled, while the others are called kinetically controlled. The reaction of 4-hydroxydiphenylmethane with the tert-butoxyl radical is an interesting example of a kinetically controlled reaction.
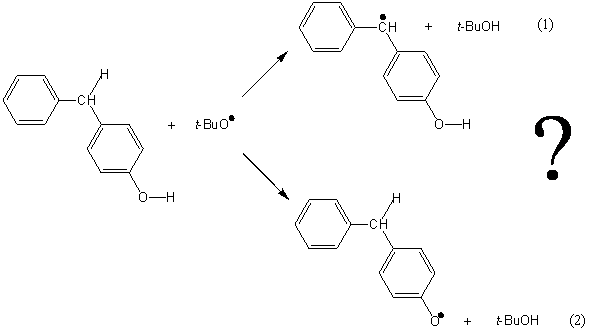
What do you think would be the outcome of this reaction? In a sheet of paper (or in this computer screen), the tert-butoxyl radical could abstract an hydrogen from either the methylenic C—H bond (reaction 1) or the phenolic O—H bond (reaction 2) in 4-hydroxydiphenylmethane. Since the C—H bond is ca. 15 kJ.mol-1 weaker than the O—H bond, based on the relative BDEs we would predict that reaction 1 would be preferred, and we would be entirely… wrong! The actual result is reaction 2: the tert-butoxyl radical selectively abstracts the phenolic hydrogen, breaking the O—H bond. Although it seems surprising that it is the stronger bond that gets broken, we should bear in mind that the strength of chemical bonds (i.e., their BDEs) is only a part of the reasons that explain chemical reactivity. In this example, reaction 2 is much faster than reaction 1 due to the lower activation energy required for O—H abstraction by tert-butoxyl compared to the abstraction from C—H. So, even though reaction 1 is enthalpically favored (more exothermic), reaction 2 is kinetically favored, and therefore this reaction is kinetically controlled.
Photoacoustic calorimetry can also be used to determine the rate of chemical reactions, thus providing a much deeper insight into chemical reactivity. This requires a development of the basic technique, by analyzing not only the amplitude but also the temporal-profile of the photoacoustic signal. The new method is called time-resolved photoacoustic calorimetry and it has been applied to the determination of rate constants of hydrogen abstraction reactions like the ones presented above, affording kinetic data in excellent agreement with those obtained by laser flash photolysis, which is the most widely used technique to probe the rates of very fast reactions.
