The photoacoustic effect and the calorimeter
Photoacoustic calorimetry is based on the photoacoustic effect, which is a general characteristic of matter: when matter absorbs light, it emits sound. The photoacoustic effect was discovered around 1880 by Alexander Graham Bell, but only a century later was the first modern photoacoustic device developed (using lasers as the light source), by IBM scientists Tam and Patel. As a calorimetry technique, PAC was applied for the first time to measure reaction enthalpies in 1983 by Kevin Peters and his group.
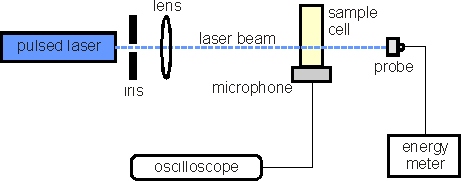
The figure above represents a very simplified photoacoustic calorimeter. The laser is used to initiate a process in the solution contained in the cell. This process can be, for instance, the cleavage of a chemical bond in the solute molecules present in the solution. The energy of the laser pulse absorbed by the solution is larger than the energy needed to break that bond, so the excess energy is suddenly deposited in the solution, causing a local temperature increase. This sudden and localized heating has the same effect as a stone being thrown in a lake: it produces a mechanical wave (sound!) that travels through the solution until it reaches the microphone. The microphone detects the sound, which is then amplified and recorded for measurement in the oscilloscope. The amplitude of the signal in the oscilloscope is proportional to the amount of heat produced, i.e., to the amount of energy not used by the photochemical reaction, denoted by ΔnrH. The amount of energy used in the photochemical reaction (ΔuH) is simply the difference between the laser pulse energy (Em) and the heat detected (ΔnrH), as indicated by the energy balance:
Em = ΔnrH + ΔuH
To obtain the reaction enthalpy (ΔrH) one needs only to divide ΔuH by the efficiency of the photochemical process, the reaction quantum yield, Φr:
ΔrH = ΔuH / Φr
One may wonder why is it necessary to develop such an elaborate method to measure heat, i.e., using a microphone. The reason is that the microphone detects the heat produced by very fast processes, typically in the range of a microsecond or less. This makes possible to probe the thermochemistry of very fast reactions, not amenable to classical instruments, like reaction-solution calorimeters. PAC is therefore a choice technique to study, for example, the energetics of reactions involving free radicals.
